News provided:
July 31, 2023, 4:41 PM EDT
Introduction
In Clinical Trials, buzzwords like "artificial intelligence," "machine learning," and "predictive analytics" are common industry terms that can be confusing without a comprehensive understanding of their application. Moreover, these terms are often tossed around carelessly without considering the actual value they provide.
Clinical research is not immune to these buzzwords being misused to describe all sorts of technological advancements. Ultimately, the real value of these concepts lies in how they serve your business and, specifically, your clinical trial. This article discusses the benefits of leveraging advanced analytics approaches in global clinical research.
What is “Clinical Trial Analytics”?
In simple terms, “clinical trial analytics” is collecting and analyzing data from clinical trials to understand clinical trial performance for faster, safer, and higher-quality research. It involves taking large amounts of data from clinical studies, organizing it into meaningful information, and extrapolating insights to inform better decision-making. Clinical Trial Analytics can and has also been described as informatics, AI, Machine Learning, or Big-Data in Clinical Research.
Why Now?
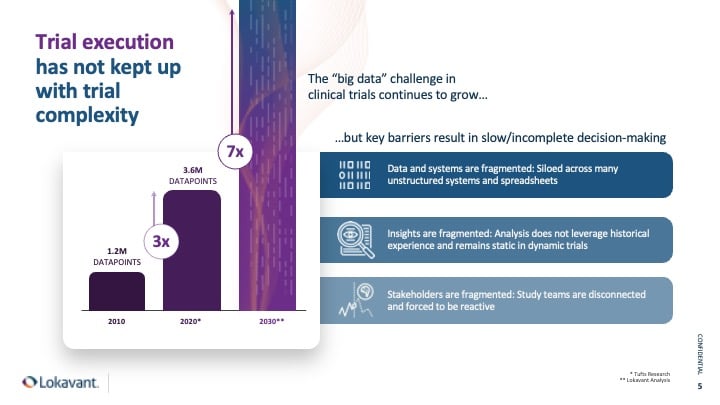
Clinical trial data has become increasingly complex in the last decade, with a three-fold increase in complexity. This increase in trial data results in fragmented data, fragmented insights, and fragmented stakeholders – leading to collaboration difficulties and unmanageable amounts of data.
Research by Tufts in 2021 has shown that Phase III trials now have over 3.6 million data points. This complexity creates significant inefficiencies in drug development, forcing teams to scramble to operate in a cost-constrained environment. To combat these challenges and create trial efficiencies, teams must leverage analytics modeled by high-quality, clean data they can trust.
To add more fuel to the fire, our analysis at Lokavant suggests that trial data complexity will continue to grow exponentially, reaching a 7x increase by 2030.
Why care? The power of Predictive Analytics
Imagine having real-time access to your clinical trial data and metrics from all systems in a centralized dashboard - from individual trials to your entire portfolio. Not only to monitor trial progress but also to see and receive alerts and signals of potential risks and compliance issues while helping you pinpoint the root cause of any problems.
This is what clinical trial analytics offer, a progressive process that can help you see what is happening in your trial (Descriptive Analytics), then enable you to understand why something is happening in your trial (Diagnostic Analytics), and finally allow you to forecast what is likely to happen in your trial (Predictive Analytics).
Overview: The evolution of analytics in clinical trials
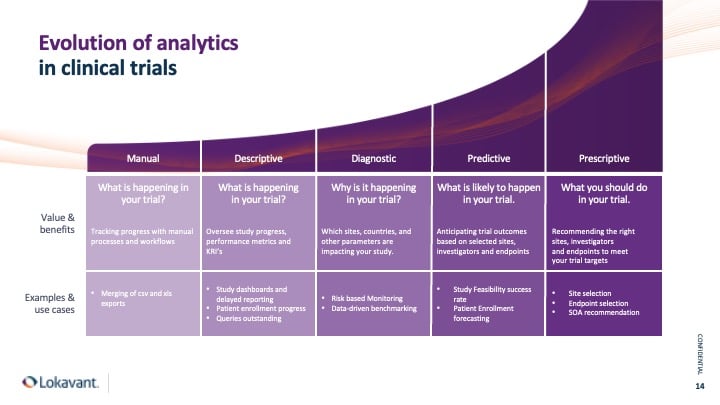
The adoption and implementation of analytics is a progressive process.
In clinical research, descriptive analytics helps us understand what has happened in the past.
Diagnostic analytics, on the other hand, helps us understand why it happened.
And moving up the analytics ladder, we have predictive analytics, which predicts what might occur in the future, followed by prescriptive analytics, which recommends the best course of action based on those predictions.
Although many clinical trials have achieved descriptive analytics to track progress, there is untapped value in leveraging trial data in unique and innovative ways. Clinical research teams can achieve greater efficiency and success by adopting a comprehensive analytics approach at every stage of the clinical trial process.
Watch this video to learn more about the evolution of Clinical Trial analytics.
Benefits: Using AI, ML, and advanced analytics in Clinical Research
Integrating AI, ML, and Advanced Analytics in clinical trials presents numerous benefits. From our experience, we see key advantages of using AI & ML in Clinical Research to help:
Improved Patient Recruitment and Retention
AI and ML can assist in identifying potential patients for trials and predicting the likelihood of their enrollment and continued participation. These technologies can significantly enhance site selection and participant recruitment and retention rates in clinical trials by leveraging vast amounts of data from sources like electronic health records (EHRs) and genomic databases. This leads to faster and more efficient trials, reducing the time and cost of bringing new treatments to market.
Proactive Clinical Trial Risk Management
Leveraging AI and ML to assist with Risk-Based Monitoring (RBM), clinical researchers can proactively manage potential risks to help identify new Key Risk Indicators (KRIs) and establish thresholds based on therapeutic area and geography. Using advanced analytics, researchers can anticipate and mitigate potential issues that could impact the performance of a trial. We’ve seen that proactive management of clinical trial operations leads to reduced protocol deviations, increased protocol adherence, and reduced patient drop-out.
Study Planning and Feasibility
One of the most impactful areas to leverage AI and ML is in the planning and design phase. By using AI/ML, researchers can take a data-driven approach to study planning and ultimately enhance the quality and performance of their trial by selecting the most appropriate sites, endpoints, and protocol parameters.
Efficient Resource Allocation
With the help of advanced analytics, clinical trial managers can better predict and manage the resources required for a trial. This leads to more efficient use of time and resources, potentially reducing the overall cost of the trial. Moreover, efficient resource allocation can also help improve patient retention and recruitment in clinical trials, a key contributor to reducing the cost and timeline of drug development.
Oversight and Compliance
AI and ML can significantly improve the integrity and compliance with Good Clinical Practice (GCP) standards by surfacing valuable data and insights to study teams that oversee the safety and compliance of clinical trials. The ability to extract insights and focus study teams can create human-in-the-loop best practices and keep highly trained humans involved in all decision-making processes without adding administrative burden or overwhelming teams with too much data.
Using Analytics in Clinical Trials with examples
Descriptive Analytics
In clinical research, data integration is a critical component of success. But with the diverse nature of clinical research and varying levels of technological maturity, achieving comprehensive and unified reporting is no small feat. That's where descriptive analytics comes in and helps study teams see their study data to track progress and measure performance metrics & KRIs.
Despite a growing focus on descriptive analytics to track progress in clinical trials, challenges persist, especially for smaller biotech companies. Manual, lagging processes, a lack of a single source of truth, and difficulties harmonizing across sources and vendors underscore the necessity of more advanced, integrated analytics solutions.
Benefits of Descriptive Analytics in Clinical Trials
Descriptive analytics, which involves interpreting historical data to identify patterns and trends, provides several significant benefits for clinical trials.
- The right level of detail
Descriptive analytics help study teams get the right data at the right time to make decisions, filtering through mountains of study data to identify critical key performance indicators in real time. This provides optimal insight to help trial managers quickly understand the ongoing studies' status, identify issues, and make well-informed decisions. - Real-Time study health
Another advantage of descriptive analytics is the ability to monitor your trial in real time, including current data. Real-time understanding of the study's health facilitates immediate adjustments, enhancing risk mitigation efforts and improving trial performance. - Milestones tracking
Descriptive analytics can offer a comprehensive view of your milestones at the study, site, and country levels in one place. This enables you to measure downstream impact and understand the progression of the study at various levels. - Connected dashboards
Finally, descriptive analytics can help connect all areas of your trial to create a data story for your trial, including Study Start-Up, Participant Enrollment, Data Cleaning & Close Out, and Study Milestones. Connecting dashboards can enable the seamless tracking and management of different aspects of the trial and centralize key components, such as leading and lagging performance indicators, to enhance the efficiency and oversight of clinical trials.
A case-study in Descriptive Analytics
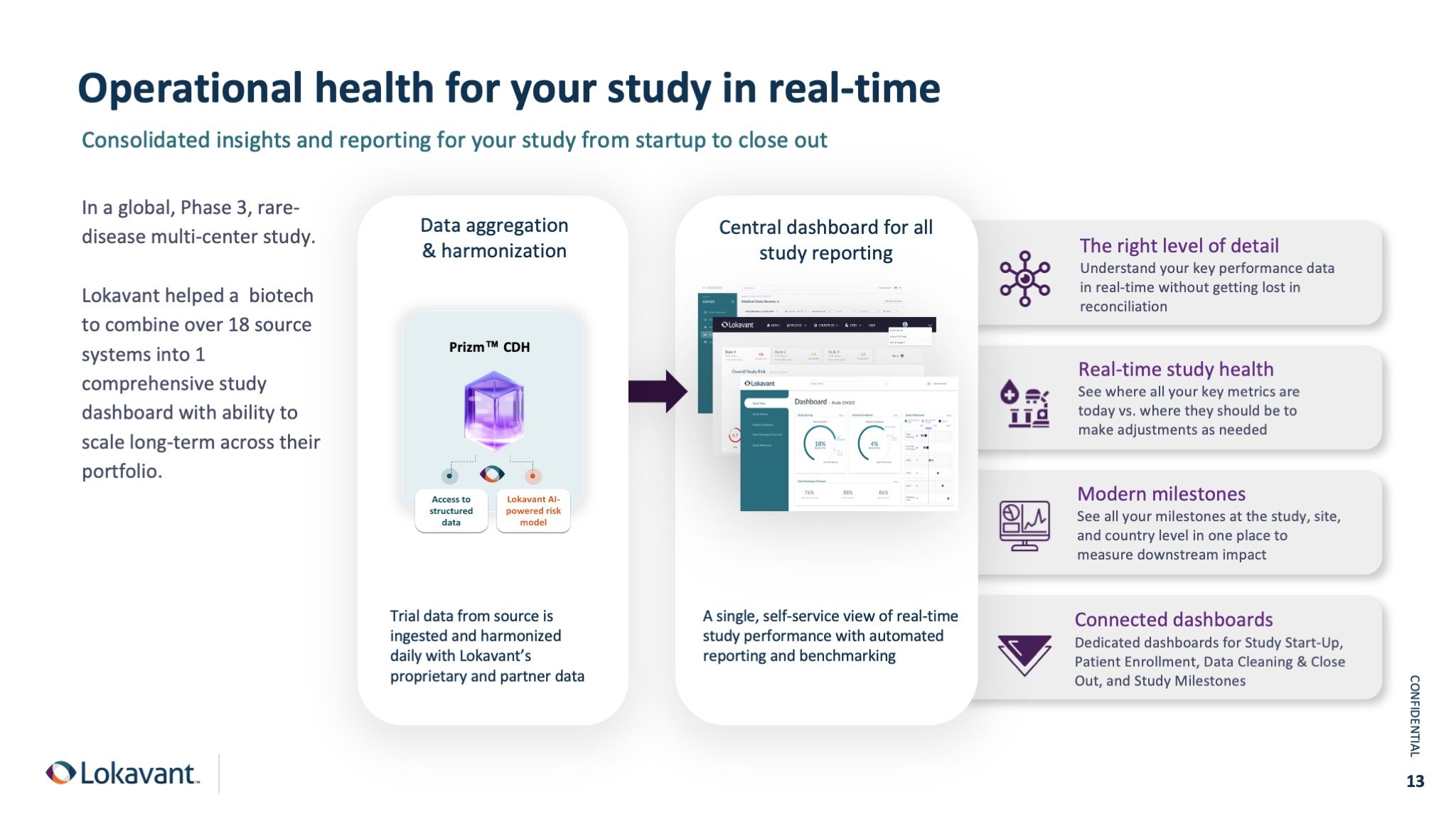
In a recent Phase 3, rare-disease multi-center study, one of the biggest burdens to the study team was consolidating all their study data from various sources into a digestible reporting system to track the study progress. Here we helped them implement Descriptive Analytics via a Clinical Data Hub that integrated and automated data integration from over 18 different source systems. The creation of a comprehensive and unified reporting structure streamlined tracking trial progress and also enabled the proactive management of study risks.
Diagnostic Analytics
Diagnostic analytics in clinical trials often leverages vast repositories of historical studies and third-party data to identify similar studies, also known as “look-alike studies.” When look-alike studies are built and utilized to develop performance benchmarks that are aligned to a specific therapeutic area, country, and phase, study teams can then use diagnostic analytics to compare their trial to the look-alike benchmarks and surface insights and to get a better understanding of why something is happening in their trial.
A case-study in Diagnostic Analytics
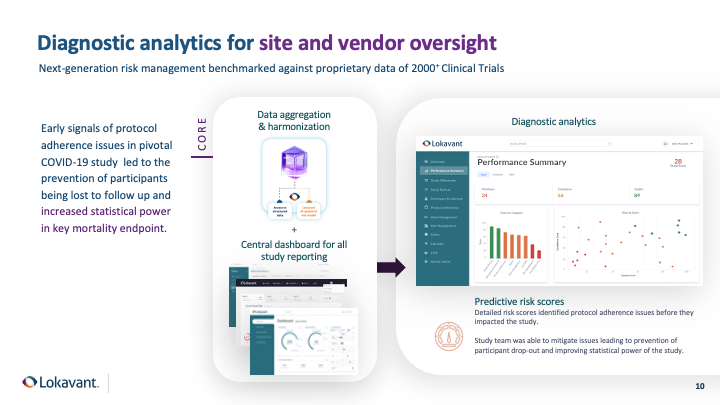
Speed was the greatest concern in a pivotal COVID-19 therapeutic study, and a trial was deployed in under 40 days. Despite the significant challenges involved and the situation's urgency, it was critical to use a diagnostic analytics approach to monitor the study and mitigate any risks.
Using a diagnostic analytics approach, the team set up performance thresholds for the protocol deviation rate based on look-alike studies. Once the threshold was surpassed, the study team was promptly informed in the early months of the study and identified abnormal protocol adherence risks compared to the benchmark that led to participants being lost to follow-up. This early intervention and immediate notification were critical in preventing significant patient follow-up loss.
By leveraging diagnostic analytics, the study team addressed these issues proactively, which helped maintain the statistical power in the key mortality endpoint.
Predictive Analytics
Predictive Analytics is the next stepping stone in applying analytics to Clinical Research. You can’t have predictive analytics without first doing the core work of the previous analytic paradigms we just discussed; however, value and insights become exponentially more exciting.
With predictive insights, clinical researchers can anticipate trial outcomes and performance in advance, leading to radical improvements in areas such as patient enrollment, retention, and compliance.
Predictive enrollment forecasting and risk mitigation
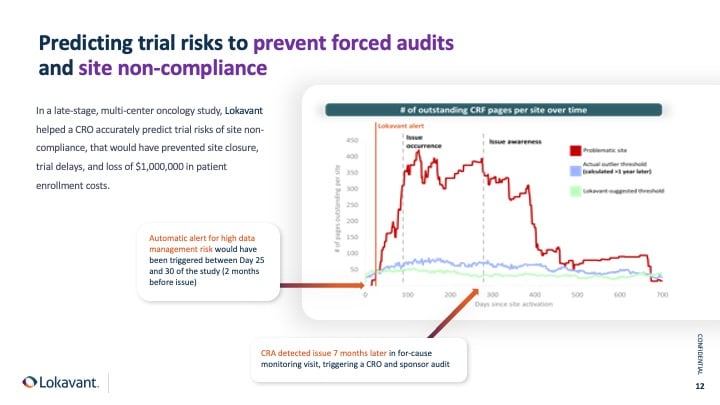
Predictive analytic models, such as enrollment forecasting models, are increasingly used. When re-forecasted frequently, these models can identify potential enrollment issues early, allowing proactive measures to mitigate any upcoming problems.
For instance, consider a global, phase-two multi-center study. Within the first month of the study, the research team was informed that it was impossible to achieve the planned enrollment target by the recruitment window. And this early insight enabled the team to take immediate action, leading to significant cost savings and transparency in the trial.
These savings were realized specifically by being able to plan from the start accurately and avoiding multiple change orders due to timeline extensions and additional site requirements.
In today's dynamic clinical trial environment, there is no clear separation between study planning and execution. Therefore, agility and the ability to continuously optimize operational plans by adjusting site, country, and investigator settings are crucial factors in ensuring success.
This scenario illustrates how using predictive analytics in patient enrollment models can enable more proactive and responsive management of clinical trials.
Challenges in Implementing Analytics and Informatics in Clinical Trials
While the benefits of applying advanced analytics and informatics in clinical trials are undeniable, several challenges can impede their successful implementation. It's important to acknowledge these obstacles and develop strategies to overcome them. From our experience working with clients globally, we see the following as the biggest obstacles to implementing AI & ML in Clinical Trials.
- Data Aggregation - An Operational Challenge
The first and perhaps most significant challenge lies in data collection, integration, and analysis. Clinical trials generate an enormous amount of data, but much remains siloed within different systems and formats, causing a significant operational challenge in implementing advanced analytics due to the lack of a single, reliable source of truth. The resulting confusion hampers understanding and control of what's really going on in the trials. Gathering a well-powered, representative data sample from previous studies in the same therapeutic area, countries, and phases as the current study is daunting. Ensuring that the collected data is high-quality and fit for purpose is a hurdle in itself. In addition, integrating disparate data into a single, coherent dataset that can be analyzed effectively is an ongoing operational challenge. - Building Trust in Analytics - Ensuring Credibility and Transparency
For analytics to be effective, clinical trial teams must trust the output. Building trust is a multi-step process that begins with clear and transparent communication about how AI, ML, and advanced analytics work. It's critical to explain the methodologies, algorithms, and data sources used in an accessible and digestible manner. This can involve simplifying complex technical jargon and demystifying the 'black box' nature of AI and ML models.
Moreover, consistently reliable performance is key to developing trust. Whenever analytics applications accurately predict trial performance or reveal insights that drive decisions, it reinforces their value and builds confidence in their capabilities. Furthermore, providing training to clinical trial teams can empower them to understand and effectively use these technologies, further bolstering trust. - Demonstrating ROI and Justification - Evidence-based Change Management
Demonstrating the return on investment (ROI), the most persuasive argument for change, from implementing analytics in clinical trials is crucial for driving acceptance and adoption. This involves showing how analytics can enhance efficiency, reduce costs, and improve trial outcomes.
Real-world case studies where analytics have successfully improved patient recruitment, increased retention rates, enhanced data quality, or driven other meaningful performance outcomes can serve as powerful testimonials. Quantifying the benefits in terms of time saved, reduced costs, or improved trial data quality can also be beneficial. Building a robust evidence base is thus key to justifying the adoption of these technologies and fostering change management.
By systematically addressing these challenges, we can improve the acceptance and adoption of analytics in clinical trials and enhance their effectiveness and potential impact.
Final thoughts
As medicine and therapies continue to innovate in science, trial performance and operations must continue to evolve to meet newer models of trials and science. Embracing advanced analytics, AI, and ML is more than a strategic advantage – it's necessary. From improving recruitment to predicting trial performance, the potential benefits are truly transformative.
From our experience, introducing these technologies into your studies operations might feel daunting, and you may have more questions. That's why we encourage you to schedule a Lunch and Learn with our team.
During this session, we will dive deeper into your trials' specific challenges and explore how advanced analytics can be tailored to your unique needs. It's a no-obligation opportunity to learn, ask questions, and reimagine the future of your clinical trial strategy.
Fill out the form below to schedule your Lunch and Learn session. Let's journey together toward a more efficient, effective, and patient-centered future in clinical trials.
Learn More
Schedule a Lunch & Learn
FAQ Section
What are AI, ML, and Advanced Analytics in the context of clinical trials?
Artificial Intelligence (AI) and Machine Learning (ML) are subsets of advanced analytics that use algorithms to recognize patterns in data, learn from them, and make predictions or decisions. These technologies can help in clinical trials, such as identifying potential trial participants, predicting trial performance, and monitoring trial risks.
How can AI and ML be used to optimize the recruitment process in clinical trials?
AI and ML can be used to analyze vast amounts of data from electronic health records (EHRs), genomics databases, and other sources to identify patients who may be eligible for specific trials and help optimize site selection. These technologies can also help predict which sites are more likely to enroll and retain participants in a trial, improving recruitment and retention rates.
Can AI and ML help reduce the high attrition rates in clinical trials?
Yes, AI and ML models can help predict patient attrition, a significant challenge in clinical trial execution. By understanding the key features and at-risk sites influencing patient drop-out rates, trial sponsors and CROs can anticipate and mitigate potential retention risks, thus improving patient engagement and accelerating the delivery of new therapies.
What are the potential benefits of using AI and ML in clinical trials?
Clinical trials can leverage AI and ML to improve the quality and efficiency of data collection, enable proactive risk management, increase the speed and accuracy of patient recruitment, and predict trial performance. These technologies can lead to better and more efficient clinical trial performance.
How can AI and ML integrate with Risk-Based Monitoring (RBM) in clinical trials?
AI and ML can play a significant role in RBM by predicting potential risks and outcomes, leading to more effective risk management strategies. They can help identify Key Risk Indicators (KRIs) and establish thresholds for these risks, enabling proactive management of risks that could impact trial performance.
What ethical considerations are involved in using AI and ML in clinical trials?
Ethical considerations include protecting patient data, consent for data use, data bias, and the transparency of AI and ML algorithms. Data Monitoring Committees (DMCs) often oversee these ethical aspects, working closely with investigators and sponsors to ensure the safety of participants and the integrity of trial data.

