News provided:
July 31, 2024, 9:12 AM EDT

________________________________________________________________________________________________________
In our recent deep dive into the clinical trial processes of top-10 pharmaceutical companies, we discovered something eye-opening: traditional site selection methods and feasibility processes are not only outdated but are also significantly impacting trial success rates.
Here’s the case-study:
Before the advent of advanced AI tools like Spectrum™, pharma companies primarily relied on manual processes and a biased US-centric approach for their trial sites. This method, while seemingly logical due to regulatory and operational conveniences, posed severe risks of over-concentration and inefficiency that ultimately leads to delays and other trial challenges.
We might wonder, why do we stick to this model?

But with Spectrum, this game has changed.
The Challenge
A top-10 pharmaceutical Sponsor faced a daunting task: identify the most suitable sites for a critical phase III Obstructive Hypertrophic Cardiomyopathy(oHCM) trial.
With a target of 500 participants spread across 250 sites, a 730-day enrollment window, and a 365-day site activation period, the stakes were high. Traditional methods left them heavily reliant on US sites, which posed significant risks, including over-concentration and extended timelines.
The Analysis
Spectrum completed a comprehensive, data-driven feasibility analysis and leveraged cutting-edge AI to create two distinct scenarios:
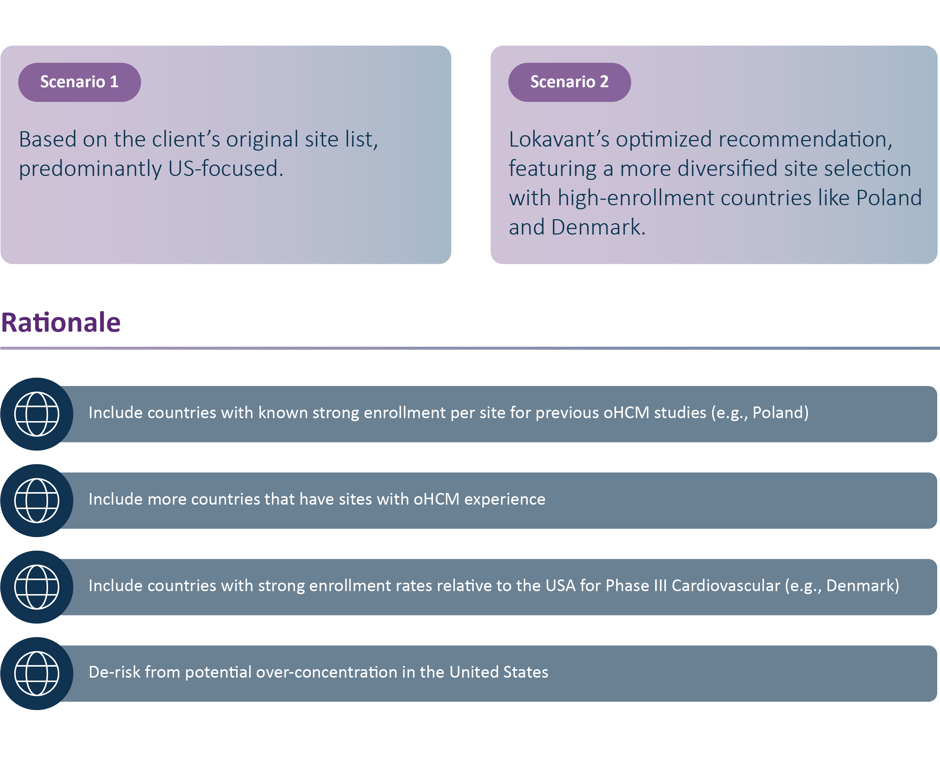
The Execution
Our machine learning model didn’t just skim the surface; it dove deep into both proprietary and public historical data, predicting trial timelines with remarkable precision. This endeavor was not just about crunching numbers but about harnessing the power of Real-World Data (RWD) and machine learning to transform clinical trial operations. Here’s what we found:
 The Impact
The Impact
The results were both clear, and compelling:
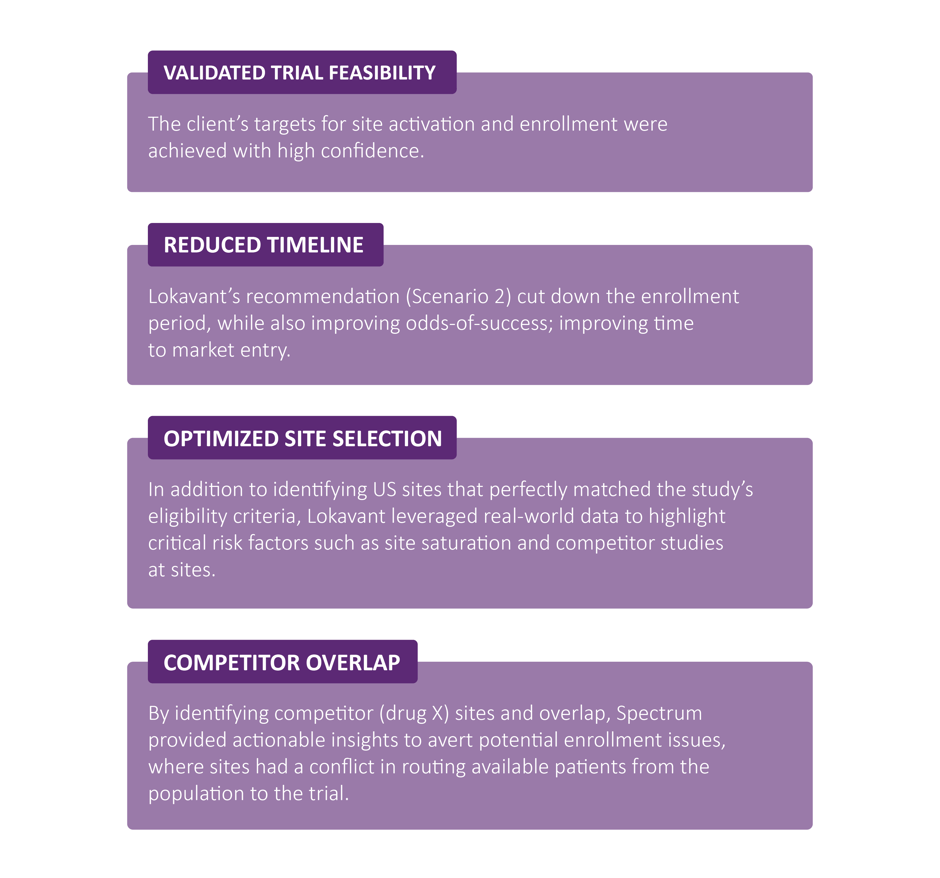
By integrating Spectrum’s AI-driven insights, this study sponsor didn’t just follow the usual path; they blazed a new trail in clinical trial feasibility.

