Published on:
April 16, 2021, 9:00 AM EDT
Lokavant was launched by Roivant Sciences, a healthcare company focused on applying technology to drug development, just a few months prior to the U.S. shutdown order at the start of the COVID-19 pandemic. With limited access to trial sites during the shutdown, and as virtual trials continue to proliferate, Lokavant’s technology and capabilities—remote trial oversight, predictive risk monitoring, and data-driven trial planning—have become even more critical to ensure effective trial execution.
The pandemic has emphasized how much is at stake if the inefficient status quo in clinical development is maintained. If we are committed to bringing meaningful therapies to patients faster and cheaper, clinical operations must evolve to meet the needs of patients and stakeholders in today’s world.
Clinical trial operations are broken. Consider this: one in six trials fails today because of preventable quality issues—problems that have nothing to do with the safety or efficacy of a potential therapy. Many drugs that do go on to receive regulatory approval are marred by delayed timelines and budget overruns; indeed, the average cost to develop an approved drug has more than doubled to $2.6 billion over the last decade.
The enterprise consequences of these operational failures are tighter margins and a damaged bottom line for sponsors and CROs. Those who suffer most, however, are the patients who these companies seek to serve, who have access to fewer treatment options and are burdened by higher drug prices.
Why are today's clinical trials inefficient? Because data is grossly mismanaged across the industry:
- Data is fragmented across different repositories, sometimes spanning up to 25 source systems within a single study, leaving key endpoints divided across siloed teams and reports. Related data is collected in different formats at different times, and it is still common to spend significant time and resources to manually verify data transcriptions from paper to digital formats. This makes it extremely challenging to process, analyze, and interpret trial data in a meaningful way.
- Institutional silos stemming from misaligned incentives, and a lack of integration of historical and concurrent trial views, preclude clinical trial data from being effectively connected across studies. As a result, most trials cannot easily access and benefit from insights in historical study data on similar compounds, indications, and patient pools—replicating the operational errors of analogous studies as emerging risks are overlooked.
- Data innovation is outpacing operations capacity, and not due to the fault of operators. Data variety, volume, and velocity can overwhelm under-resourced teams, resulting in missing and delayed data entries that go undetected. While critical to move the field forward, the rapid adoption of hybrid and virtual trials is exacerbating these challenges, giving form to new, multitudinous, and complex data sources that refresh at even faster rates.
Due to these challenges and inefficiencies, by the time trial data is aggregated in a way that is useful to understand, it is usually far too late to apply insights to a trial. Human analysis reveals errors days after they occur, at best, and often overlooks early indicators of risk in today’s increasingly complex studies. Current technological solutions do not offer much beyond static statistical monitoring, typically uncovering mission-critical errors related to trial startup, enrollment, follow-up visits, etc. some time after they have occurred and compounded. Teams are left chasing the source of issues rather than proactively identifying and mitigating them, resulting in additional costs and potentially detrimental delays.
Moreover, study designers and planners are often blind to the real-world impacts of study parameters, resulting in impractical study timelines and budgets. In the absence of systematically collected, aggregated, and analyzed data, trial operators often rely on data from a few recent studies or on outdated benchmarks of lookalike trials.
How does Lokavant address these challenges?
The best way to mitigate operational error in trials is to enable clinical teams to be proactive rather than reactive at every stage in the process. We envision a world where operators no longer have to respond to quality issues after they’ve surfaced, wasting critical time and resources on tedious and complex operational matters. That means reversing the paradigm so that the influx of data is no longer a burden for clinical operators, but instead enables teams to make informed decisions beginning at the trial’s planning phase—facilitating error-free execution all the way through study completion.
Here’s how we do it:
- A single source of truth. We believe that data mismanagement is the primary source of operational error. We address this by centralizing and integrating data—wherever it sits and regardless of format—into a warehouse defined by a single common language. Anyone on any team should be able to access and understand the status of their operations in real time. Our systems ingest and refresh data from original sources in real time, creating a cohesive, harmonious data structure with the most up-to-date information.
- Forward-looking insights about what will happen in your study, powered by historical data. We have observed firsthand that there is a wealth of historical operational data at most pharma companies and CROs, but few are accessing this information after a trial is complete, let alone using it to better plan and execute their future trials. This is because it takes significant data science expertise and industry context to unlock meaningful insights from this data. From day one, we recognized this challenge and have brought in a world-class team of data scientists and engineers from within and beyond the industry to tackle these challenges. Just one year later, we’ve ingested over 2,000 studies across all phases, geographies, and major therapeutic areas. Our solutions are already changing the way study operators tackle core problems. Some examples include:
· Enrollment: Moving from straight-line estimations set at the beginning of a study (if at all) to dynamic. daily predictions of enrollment end dates and enrolled subject sat any point in time, with 95% accuracy.
· Site compliance: Alerting clinical operators to noncompliant sites by modeling site-level quality data and outcomes, informed by analysis from nearly 100,000 global sites.
· Risk-based monitoring plans: Prior to study execution, helping study monitors set the right thresholds and QTLs by predicting the relative importance of over 20 different study risks.
This is just the beginning. With each datapoint from each trial we ingest, our models become more effective, augmenting our ability to support clinical operators. - Designed for ease of use by clinical operators. As former sponsors ourselves, we have the experience of working with dozens of clinical data solutions on the market, which largely fall into two buckets: (1) designed decades ago for IT users, or (2) built more recently, but for statistical experts. In fact, more than one solution provider suggests hiring a full-time consultant to interpret the results of their software. While these stop-gap measures were adequate in the past, we recognize that the only way to achieve our vision is to build technology that is not just usable, but simple for clinical operators to use. Before we launched Oversight—our first product—our team spent hundreds of hours with project managers, monitors, data managers, quality teams, and other operators to ensure that any insights we provide are timely, valuable, part of the right workflow, and easy to understand without a Ph. D. in statistics.
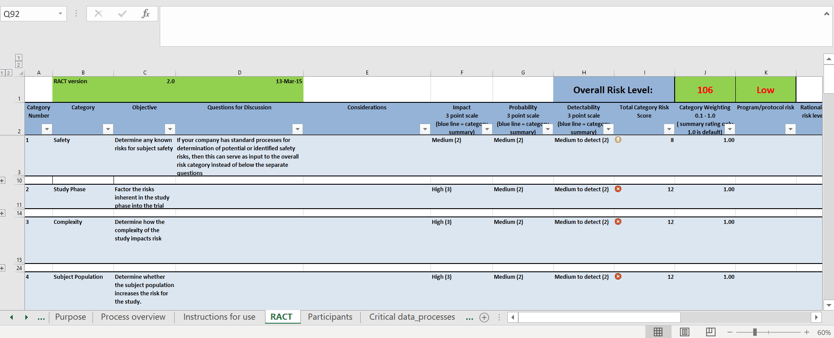
A sample Risk Assessment and Categorization Tool (RACT) template in Microsoft Excel (compare with Lokavant’s cloud-based dashboard below)
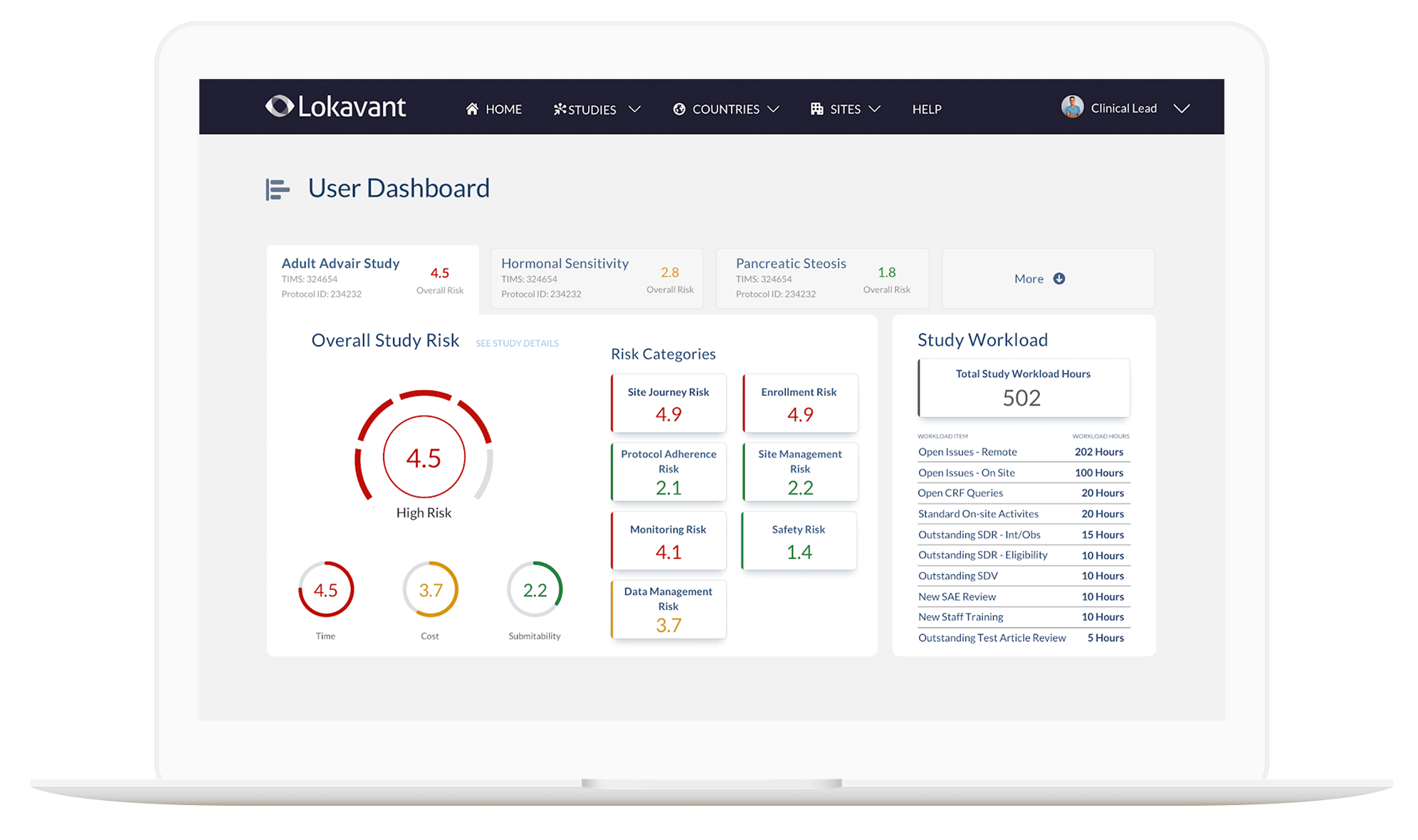
Lokavant Oversight application dashboard home page
We believe that these efforts will fundamentally transform the way that clinical trials are run. Powering actionable risk-mitigation insights as soon as the first patient enrolls in a trial, our industry-leading data repository and data standardization capabilities enable users to make evidence-backed decisions that prevent errors from occurring and compounding. We believe that this will:
- Deliver safer drugs to market faster. Robust insights and risk-mitigation opportunities will drastically de-risk R&D, contributing to faster and safer drug development
- Enable clinical teams to focus on the patient. Tedious operational issues divert time and already-strained resources away from addressing challenges directly related to the well-being of the patient. Our solutions allow teams to systematically uncover and prioritize matters related to safety and efficacy, rather than focusing on one-off errors.
- Tie drug cost to value. On-site monitoring visits and activities related to monitoring cost up to $580 million during the life of an approved drug, which translates to a total of $30 billion each year across the industry. More accurate, actionable risk mitigation insights will cut down these bloated costs, helping to tie drug prices to the real value created for patients.
In a rapidly evolving, tech-driven world, data mismanagement is a mounting barrier to efficient and effective clinical development. By combining the most rigorous data and analytics with existing human experience, Lokavant is enabling a world where no trial fails, is delayed, or is over budget because of preventable operational issues—to bring cheaper drugs to patients in need more quickly and efficiently.
Are you a sponsor or CRO running a clinical trial? If so, we’d love to hear from you. Get in touch at contact@lokavant.com.
P.S. Want to join the mission? We are looking to expand the team with the best and brightest people who are passionate about fundamentally changing the way clinical trials are run.
Check out our open positions at https://www.lokavant.com/careers.

